

STUDIES & RESULTS
Weight Loss Direct believes so much in the power of our weight loss program that we have undertaken two separate studies. One is an Institutional Review Board approved Double-Blind, Randomized, Placebo-Controlled, Parallel Trial, as well as, a Prospective, Longitudinal Cohort Study of the Weight Loss Direct Classic Weight Loss Program.
The results of the prospective cohort analysis are listed below, which not only included the best results but even the least results. This analysis demonstrated that the Personalized & Supervised Weight Loss Direct Classic weight loss program is very effective as you can see from the results below.
The prospective cohort analysis demonstrated that the top 25% of Personalized & Supervised Weight Loss Direct Classic weight loss program participants lost an average of 38.7 pounds.
The overall average weight loss of the Classic Weight Loss Direct program was 26.2 pounds*
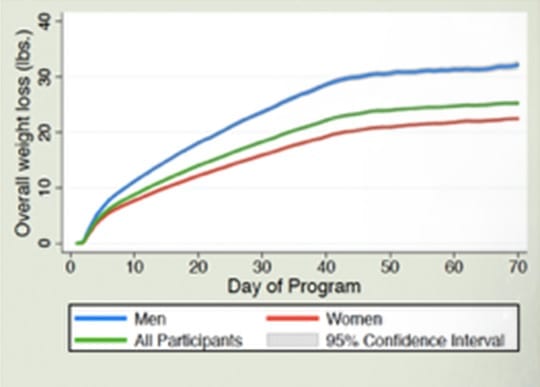
Both studies demonstrated that the average user not only sustained their weight loss but continued to lose weight after the weight loss phase of the program. The average user in the Prospective study lost over 3 lbs., while the average user of the RCT lost 1.4 lbs. after the weight loss phase of the program.
The IRB approved Randomized Controlled trial demonstrated that participants of the non-personalized & unsupervised Classic weight loss program safely lost an average of 6.13 pounds in the first 21 days.*
Prospective Longitudinal Cohort Analysis
Average, minimum, and maximum total weight loss (lbs) by quartile and gender
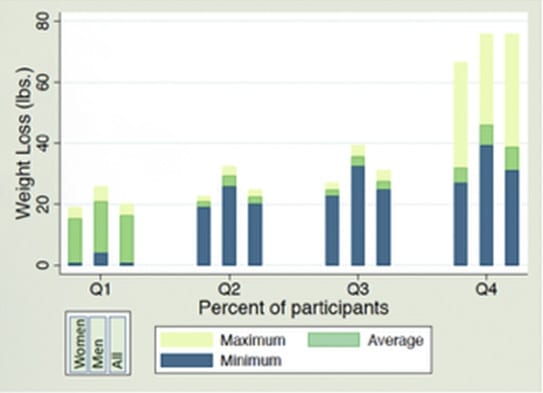
Top 25% of Participants
| Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
|---|---|---|---|
| Men | 46.0 lbs average men | 39.2 lbs. | 75.8 lbs. |
| Women | 31.9 lbs average women | 26.9 lbs. | 66.4 lbs. |
| Total (All) | 38.7 lbs average overall | 31.1 lbs. | 75.8 lbs. |
50-75% of Participants
| Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
|---|---|---|---|
| Men | 35.7 lbs average men | 32.5 lbs. | 39.2 lbs. |
| Women | 24.7 lbs average women | 22.7 lbs. | 26.9lbs. |
| Total (All) | 27.6 lbs average overall | 24.8 lbs. | 31.0 lbs. |
25-50% of Participants
| Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
|---|---|---|---|
| Men | 29.3 lbs average men | 25.8 lbs. | 32.4 lbs. |
| Women | 20.9 lbs average women | 19.0 lbs. | 22.7 lbs. |
| Total (All) | 22.5 lbs average overall | 20.2 lbs. | 24.8 lbs. |
Bottom 25% of Participants
| Gender | Average Total Weight Loss | Minimum Total Weight Loss | Maximum Total Weight Loss |
|---|---|---|---|
| Men | 21.0 lbs average men | 4.0 lbs. | 25.7 lbs. |
| Women | 15.3 lbs average women | 0.6 lbs. | 19.0 lbs. |
| Total (All) | 16.3 lbs average overall | 0.6 lbs. | 20.1 lbs. |
Prospective Longitudinal Cohort Case Study Data comes from first-time client submitted data to a third party for tracking of daily weight loss and progress through the Weight Loss Direct weight loss programs. All results are accurately and transparently presented.
IRB Approved Randomized Controlled Trial was sponsored by Weight Loss Direct and conducted by an independent contract research organization (Global Clinicals, Inc., Los Angeles, CA).
STUDY COMPARISON
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
GOALS
To assess the efficacy of the personalized & supervised 40-Day weight loss program
To determine short-term weight loss sustainability of the personalized 40-day weight loss program
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
GOALS
To assess the efficacy of the nonpersonalized and unsupervised 40-Day weight Loss Program
To determine the safety of the 40-Day weight loss program by utilizing blood work, vitals & assessment questionnaires.
To assess medium-term weight loss sustainability (2.25 months after the weight loss phase)
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
PERSONALIZED
The weight loss program was personalized and optimized for each individual user regarding both foods and supplementation
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
NONPERSONALIZED
The protocols were the same for each user. Each user was given the same list of foods and were all placed on the same 4 nutritional products.
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
SUPERVIZED
Each user was assigned a personal coach who had access to user’s daily weights. User could contact coach daily and would meet each week to review results and make adjustments to help coach and guide the user to success.
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
UNSUPERVISED
The user had four set meetings (including the 1st visit) with a researcher, whose job was merely to gather information and could not intervene or provide guidance or coaching.
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
RESOURCES AND TOOLS
The user was given a number of resources and tools including member only user portal, which provided an abundance of resources, as well as, advanced email and text support.
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
MANUAL
No resources or tools were given to the user except a “generic” manual / guide.
PROSPECTIVE LONGITUDINAL COHORT ANALYSIS
SAMPLE SIZE
The sample size of the study was large at 4,505 users
IRB APPROVED RANDOMIZED PLACEBO-CONTROLLED TRIAL
SAMPLE SIZE
The sample size of the study started at 120 participants. 60 Active and 60 control. The trial ended with 36 active participants and 33 control participants.
STUDY COMPARISON
Maximum Weight Loss Attained over 40 days, by Quartile
Cumulative Weight Loss Over Time
IRB APPROVED DOUBLE-BLIND RANDOMIZED PLACEBO-CONTROLLED TRIAL:
Lipid Panel-Change from Baseline Day 45Lipid Panel
Day 45 - BaselineActive Group
Mean (SD) N=36Control Group
Mean (SD) N=33P-value*
Cholesterol
-8.33 (20.77)
-1.45 (25.40)
0.2209
Triglycerides
-11.53 (29.81)
0.39 (38.44)
0.1528
HDL Cholesterol
-0.75 (7.28)
0.97 (10.31)
0.4307
Cholesterol/HDL
-0.08 (0.79)
-0.05 (0.52)
0.8627
LDL
-5.39 (15.84)
-2.48 (18.89)
0.4901
VLDL
-2.19 (5.99)
0.06 (7.72)
0.1778
Lipid Panel-Change from Baseline Day 21Lipid Panel
Day 21 - BaselineActive Group
Mean (SD) N=36Control Group
Mean (SD) N=33P-value*
Cholesterol
-11.72 (17.47)
-4.36 (23.74)
0.1450
Triglycerides
-4.20 (29.66)
0.54 (31.96)
0.5250
HDL Cholesterol
-2.61 (6.63)
0.18 (6.98)
0.0931
Cholesterol/HDL
0.02 (0.63)
-0.09 (0.48)
0.4305
LDL
-8.28 (14.36)
-4.97 (18.05)
0.4006
VLDL
-0.83 (5.83)
0.42 (6.02)
0.3813
Conclusion
Effectiveness of the Program:
Both studies demonstrate that the Weight Loss Direct 40-Day Weight Loss Program is effective for weight loss.
Safety:
The RCT trial utilized multiple factors including comprehensive blood work to demonstrate that the Weight Loss Direct 40-Day weight loss program resulted in safe weight loss.
Sustainability of the Weight Loss:
Both the Prospective Longitudinal Trial and the Randomized Controlled Trial demonstrated sustainability of the weight loss with the RCT showing the weight loss continued after the weight loss phase from day 45 to day 112 during which time the calories were substantially raised with the average person losing an additional 1.4 lbs. on average. The Prospective trial demonstrated that during the 21 to 30 days after the weight loss phase “comparing the set point weight to the maximum of the weights reported throughout the entire maintenance period revealed an average continued weight loss of 3.1 lbs. for women and 3.3 lbs. for men. 84.2% of women and 80.0% of men had no weight gain or continued to lose weight compared to their weight set point throughout a one-month period following the last day of the weight reduction phase of the program and after a substantial increase in calories added back to diet. 95.0% of women and 92.1% of men maintained a weight within 2 lbs. of their set point weight.”
The results of this study indicates that the Weight Loss Direct weight loss program safely and effectively effectuated weight loss results.
Weight Loss Direct has received STRONGSCIENCE Level 2 certification for efficacy and safety of its weight loss program

